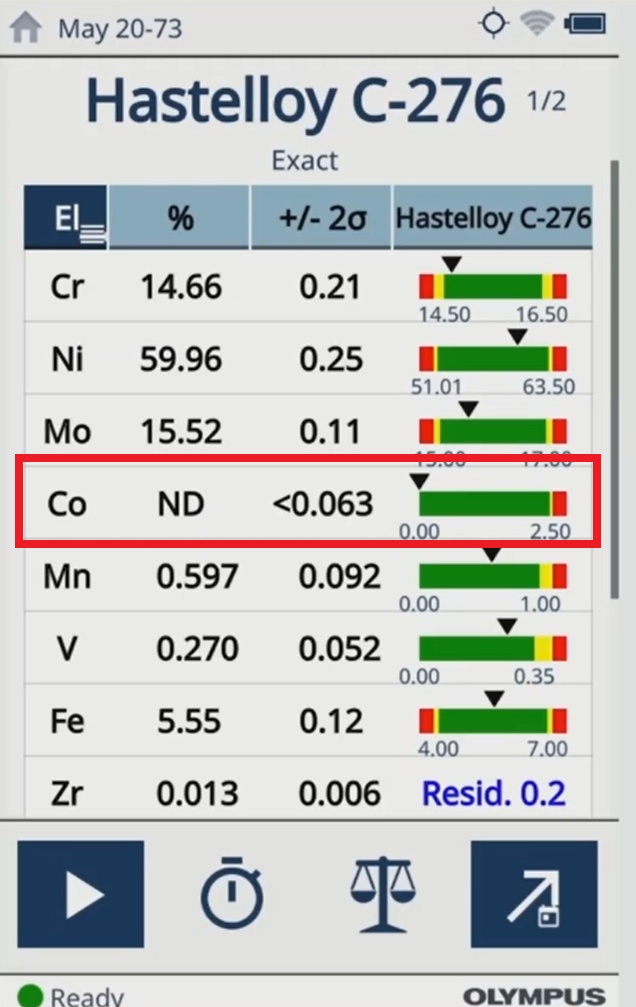
As of May 2021, the European Union Medical Device Regulation (EU MDR) 2017/745 requires manufacturers to indicate the presence of cobalt* in medical devices if the cobalt content exceeds 0.10%. This regulation is important as cobalt was identified as a chemical that may be mutagenic, carcinogenic, or toxic to the reproductive system.
The Quality Control Challenge of Cobalt
Complying with this regulation presents a new challenge to suppliers of medical parts and components: how to implement complete control over incoming goods to identify the presence of cobalt in stainless steel and nickel alloys.
Implementing 100% quality control for cobalt by producers of medical device components is recommended for several reasons.
First, it's crucial for regulatory compliance to make sure that a device's cobalt content stays below the parameters established by the EU MDR. Products that do not comply can lead to expensive product recalls and harm your corporation’s reputation.
Second, employing more cobalt than permitted can raise the cost of production. The reason is that more expensive materials might be required to meet regulatory standards.
Lastly, using premium components and thorough quality control procedures can result in the manufacturing of medical devices that are safer and more dependable, which can eventually boost client satisfaction and loyalty.
Portable X-ray fluorescence (XRF) analysis is a useful method for determining the cobalt concentration of medical devices. Portable XRF analyzers employ X-rays to excite the atoms in a sample and quantify the fluorescence that results. It’s a quick and precise way to figure out the elements present in a sample and the amount of each element present.

Vanta handheld XRF analyzer for regulatory and safety screening.
Benefits of Using Portable XRF to Identify Cobalt in Medical Devices
Here are some advantages of using portable XRF, such as our trusted Vanta™ handheld XRF analyzer, to identify the cobalt content in medical devices:
1. Speed
Portable XRF analyzers can quickly test a sample and provide results within minutes. Our Vanta analyzers can be configured to provide pass/fail results of regulated elements for efficient screening. This is especially useful for manufacturers who need to test many devices in a short amount of time.
2. Accuracy
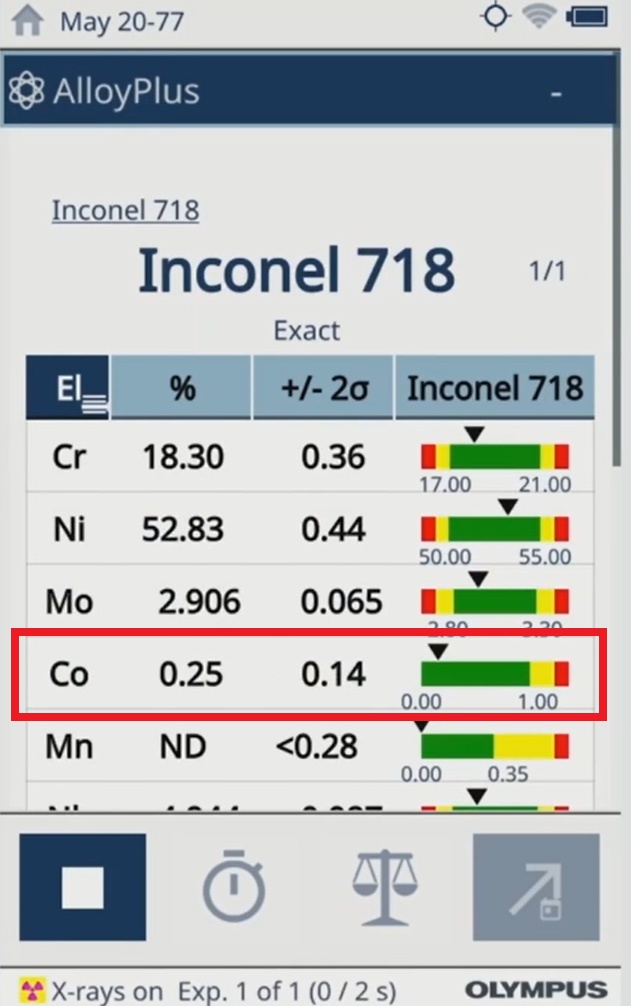
Vanta analyzers are portable XRF devices known for their accurate and repeatable results. They can detect cobalt at very low concentrations (down to parts per million). This ensures that manufacturers can accurately determine the cobalt content of their devices and meet the requirements of the EU MDR.
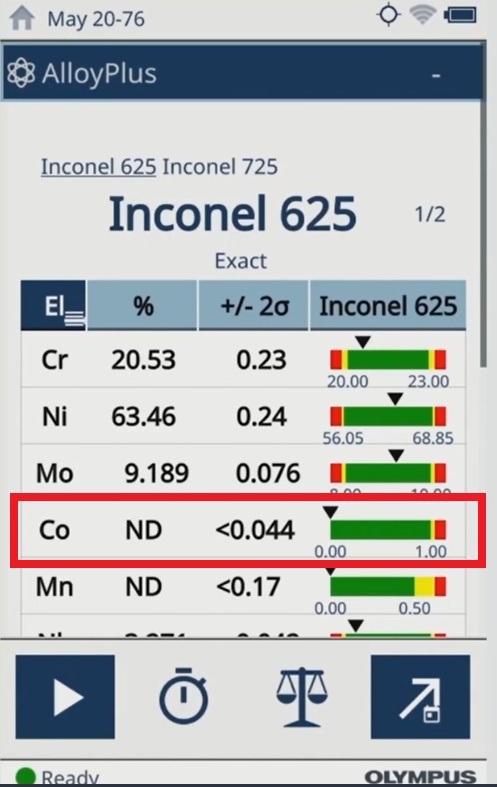 |  |
| The test results on the Vanta analyzer indicate cobalt is not detected (ND) in the medical device materials. |
3. Nondestructive
Portable XRF is a nondestructive technique, meaning it does not alter or damage the sample being tested. This is important for medical devices, as destroying a device during testing can be costly and time consuming.
4. Portable
As the name suggests, portable XRF analyzers are lightweight and easy to transport. Manufacturers can take the instruments to different locations and test devices on-site, making them a convenient and cost-effective method.
Learn More about Screening for Cobalt and Other Regulated Elements Using XRF
Portable XRF is an important tool for manufacturers to meet compliance with the EU MDR and to provide safe and effective medical devices to patients. If you have any questions about regulatory and safety screening using portable XRF, reach out to us today for expert assistance.
*Evident is dedicated to making people’s lives healthier and safer. We are committed to responsibly doing business and serving our markets in alignment with the Guiding Principles on Business and Human Rights set forth by the United Nations.
Related Content
Brochure: Control the Quality of Medical Device Materials
Screening for RoHS Substances with XRF
The Benefits of Handheld XRF for Medical Device RoHS Compliance